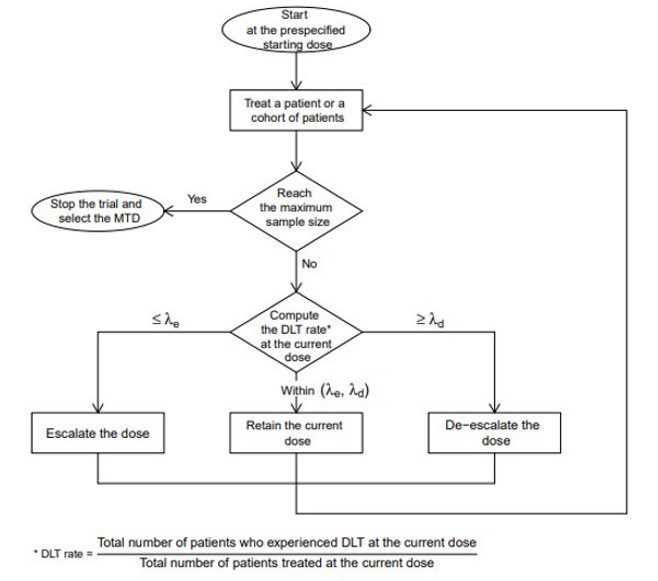
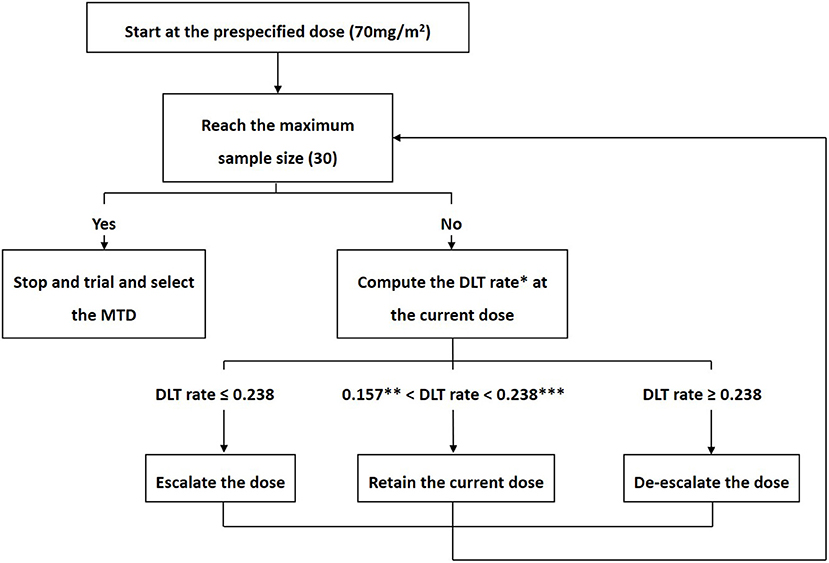
Study design for dose-escalation cohorts and DLT rate (as calculated by... | Download Scientific Diagram

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Bayesian Optimal Interval Design: A Simple and Well-Performing Design for Phase I Oncology Trials. - Abstract - Europe PMC

Exposure driven dose escalation design with overdose control: Concept and first real life experience in an oncology phase I trial - ScienceDirect

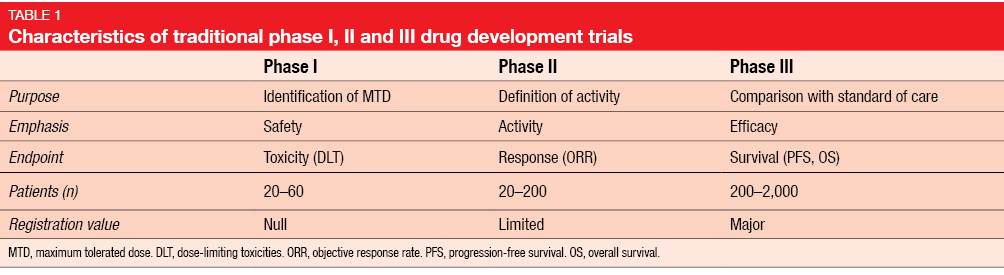
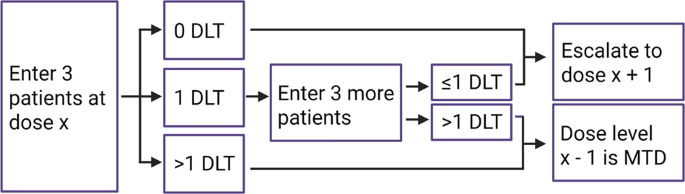
Statistical controversies in clinical research: requiem for the 3 + 3 design for phase I trials - Annals of Oncology
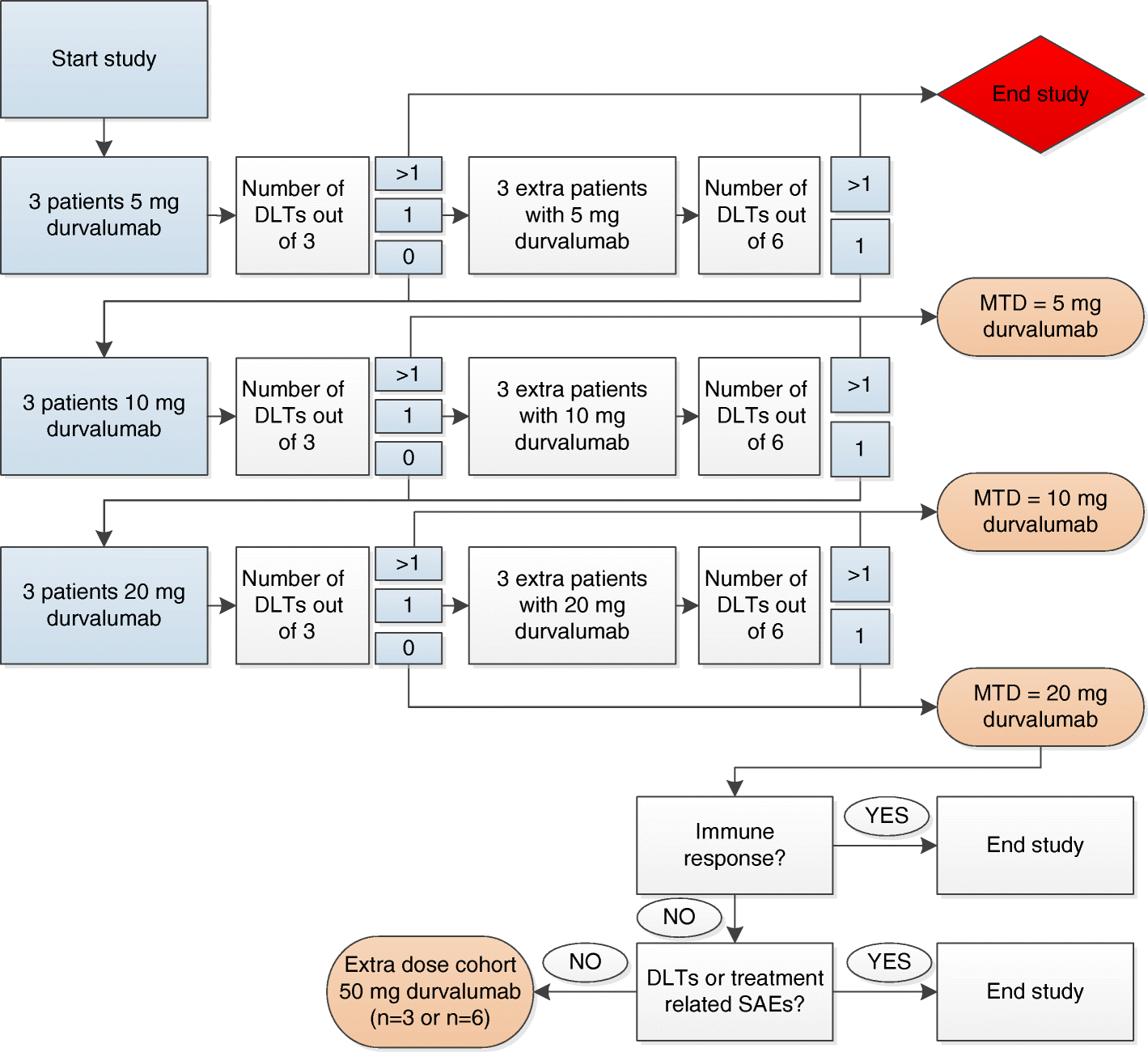
DURVIT': a phase-I trial of single low-dose durvalumab (Medi4736) IntraTumourally injected in cervical cancer: safety, toxicity and effect on the primary tumour- and lymph node microenvironment | BMC Cancer | Full Text

Safety and tolerability of bosutinib in patients with amyotrophic lateral sclerosis (iDReAM study): A multicentre, open-label, dose-escalation phase 1 trial - eClinicalMedicine
Frontiers | A Dose-Finding Trial for Hyperthermic Intraperitoneal Cisplatin in Gynecological Cancer Patients Receiving Hyperthermic Intraperitoneal Chemotherapy

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study - ScienceDirect
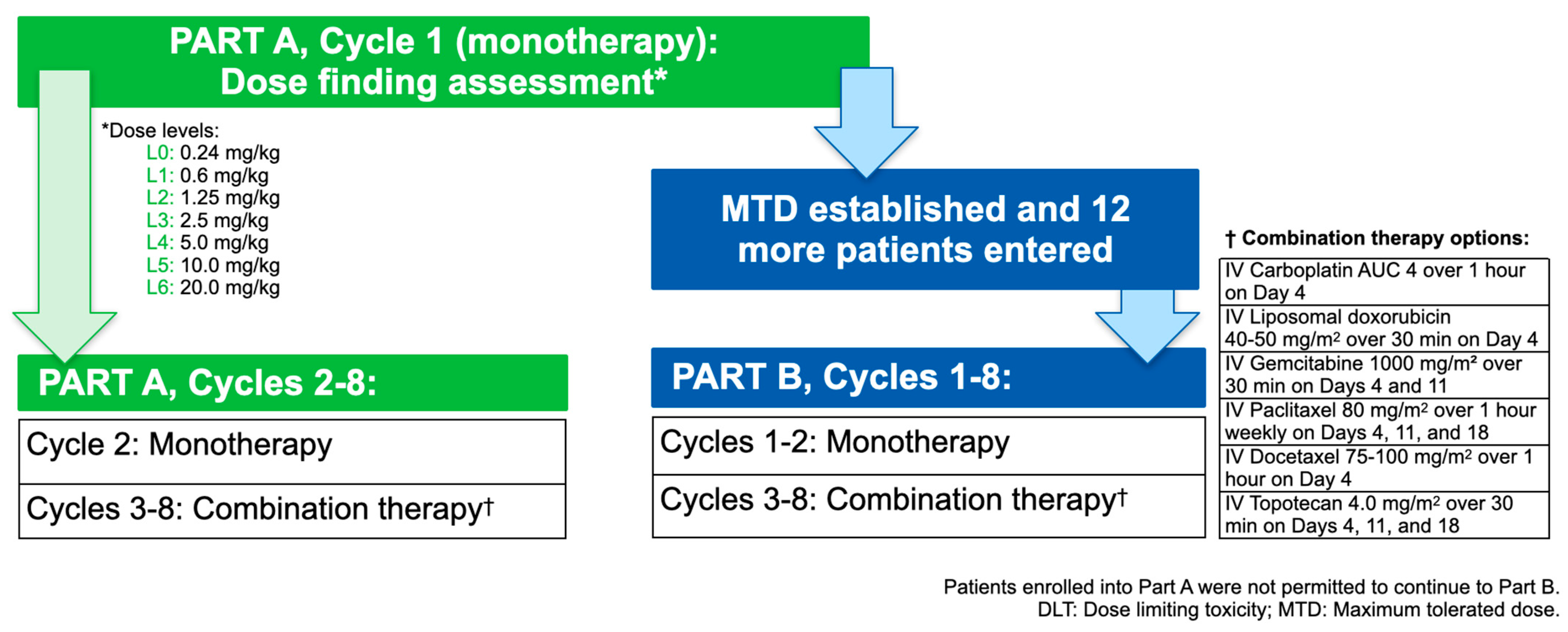
Cancers | Free Full-Text | Maximum Tolerated Dose and Anti-Tumor Activity of Intraperitoneal Cantrixil (TRX-E-002-1) in Patients with Persistent or Recurrent Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer: Phase I

Adaptive design for identifying maximum tolerated dose early to accelerate dose-finding trial | BMC Medical Research Methodology | Full Text
A Multicenter Phase I/II Study of Obatoclax Mesylate Administered as a 3- or 24-Hour Infusion in Older Patients with Previously Untreated Acute Myeloid Leukemia | PLOS ONE
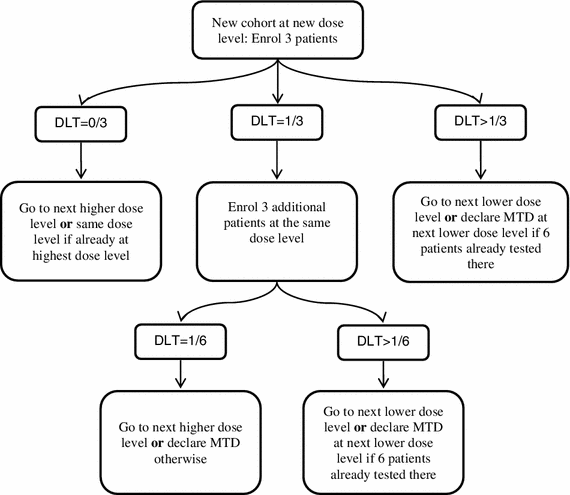
Principles of dose finding studies in cancer: a comparison of trial designs | Cancer Chemotherapy and Pharmacology

Dose-finding clinical trial design for ordinal toxicity grades using the continuation ratio model: an extension of the continual reassessment method - Emily M Van Meter, Elizabeth Garrett-Mayer, Dipankar Bandyopadhyay, 2012

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study. - Abstract - Europe PMC